Abstract
Contrast enhancement (CE) on brain non-contrast computed tomography (NCCT) is common after endovascular thrombectomy (EVT) in patients with acute ischemic stroke (AIS), but its association with clinical outcomes is not well established. The current study aimed to investigate this relationship. We retrospectively reviewed consecutive patients with acute ischemic stroke who had hyperdensity on NCCT immediately after EVT for anterior circulation large vessel occlusion (LVO) from January 2016 to December 2019. We used ASPECTS combined with volume measurement by 3D reconstruction to estimate the extent and location of CE. Multivariable regression analysis was conducted to explore the risk factors associated with clinical outcome. In this study, 113 of 158 (71.52%) anterior circulation AIS-LVO patients had hyperdensity on brain NCCT. After strict inclusion and exclusion criteria, a total of 64 patients were enrolled in the final analysis. In logistic regression analysis, CE-ASPECTS, CE volume, CE at the caudate nucleus, M4 and M6 region were associated with 3-month poor functional outcome after adjusting for confounding factors. The conventional variable model was used for reference, including age, initial NIHSS, the procedure time, stent retriever passes, recanalization status and baseline ASPECTS, with AUC of 0.73. When combined with the above-named variables (conventional variables + CE-ASPECTS + CE volume + CE at caudate nucleus + CE at M4 region + CE at M6 region), the predictive power was significantly improved, with AUC of 0.87 (95% CI 0.78–0.95). The spatial location and volume of CE on NCCT obtained immediately after EVT were independent and strong predictors for poor outcome at 3-months in patients with AIS after excluding definite hemorrhage by 24-h follow up CT.
Similar content being viewed by others
Introduction
Endovascular thrombectomy (EVT) is recognized to be an effective therapeutic strategy to restore blood flow in acute ischemic stroke (AIS) due to large vessel occlusion (LVO)1,2. Contrast enhancement (CE), defined as a visually distinct hyper-density on non-contrast computed tomography (NCCT) obtained immediately after the procedure, is commonly found with a reported incidence of 30.7–87.5%3,4,5,6,7,8,9,10. CE is believed to be due to disruption of the blood brain barrier (BBB)4,11,12,13, resulting in extravascular leakage of contrast medium, some of which may be mixed with extravascular blood.
Some studies have reported that the presence of CE was a risk factor of functional outcomes3,4,5, while others did not find prognostic value8,9,10. We argue that the controversial results may be due to heterogeneous definitions of hyperdensity, the different computed tomography equipment used after EVT, or the lack of quantitative assessment of CE. The prognostic value of hyperdensity on NCCT after EVT has not been well established and merits further investigation.
The Alberta Stroke Program Early CT Score (ASPECTS), a quantitative topographic scoring system, divides the MCA territory into 10 regions based on functional importance rather than extent, which was a simple and reliable systematic method to assess early ischemic change14,15. We hypothesized that this localization weighted method of ASPECTS is also suitable for evaluating the severity of CE. Based on the spatial distribution of CE referring to the ASPECTS system and its volumes on NCCT immediately after EVT, the present study aimed to investigate the relationship between CE and clinical outcome in patients with AIS.
Methods
Patients
This retrospective study was approved by institutional review board of General Hospital of Northern Theater Command (IRB: Y(2020)087) with waiver of informed consent. All methods were carried out in accordance with relevant guidelines and regulations. Between June 2016 and October 2019, we retrospectively screened consecutive patients with EVT from the Department of Neurology of the General Hospital of Northern Theater Command. Electronic medical records and imaging information were obtained by the hospital information system, and picture archiving and communication systems connected by a local area network. The inclusion criteria were as follows: (1) Patients with acute anterior circulation ischemic stroke with LVO who underwent EVT within 24 h of onset; (2) Prestroke modified Rankin Scale (mRS) score was less than or equal to 1; (3) There was hyperdensity on the NECT within 1 h after EVT, which was difficult to distinguish between the contrast agent and hemorrhage by two experienced neurointerventionists (J.Q and W. L). Patients were excluded for the following reasons: (1) patients who did not receive NCCT within 1 h after thrombectomy or failed to follow-up; (2) definite hemorrhagic transformation on the 24-h follow-up NCCT; (3) inadequate clinical data; (4) unqualified image due to extensive artifacts.
Data collection
The baseline characteristics were collected from the medical records, including demographics, medical history and risk factors of cerebrovascular diseases (smoking, alcohol drinking, hypertension, diabetes mellitus, and atrial fibrillation), stroke characteristics, pharmacological therapies, neuroimaging features, and procedural details. The patient’s stroke severity on admission was assessed by the National Institute of Health Stroke Scale (NIHSS).
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
Imaging analysis
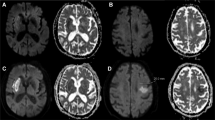
Non-contrast cranial CT (120 kV, mA/slice 400 mA, 5.0 mm axial images, the field of view 25 cm; General Electric, Boston, United States of America) was used in this study. ASPECTS was utilized to assess the spatial distribution of CE, and allotted 10 points of the territory of the middle cerebral artery. Each ASPECTS region was scored as 0 if CE was present, as 1 if there was no CE14. CE -ASPECTS and any location of CE was evaluated and recorded (Fig. 1A,B).
A 71-year-old patient presented with left-sided weakness (CE-ASPECT 6 and CE-Volume 9.041 ml). (A) and (B) Showing CE in the insula, lenticular nucleus, M1 and M2 ASPECTS regions. Thus, the CE-ASPECTS was counted as 6. (C) and (D) The three-dimensional structure of CE in the brain was reconstructed with a volume of 9.041 ml.
The CE volume was measured by automated 3D-Slicer software (Fig. 1C,D). The CT data of the patient in Digital Imaging and Communications in Medicine (DICOM) format was imported into the 3D Slicer software (3D Slicer 4.11). After adjusting the window width and window level to CT-brain, the next steps were as follows: run Editor → Threshold → Use for masking. By using the threshold range that was manually set according to the HU value of CE for masking and switching to paint effect, the pixels in which CE was present was further manually marked. Given the possible intersection of threshold ranges between normal brain tissue and CE, the individual threshold range (the maximum and minimum HU values) was set according to the HU value of CE in each patient based on Image J software to assure the reliability and reproducibility of the measurement method. Then, the three-dimensional reconstruction of the CE was realized, and the volume of the CE was calculated automatically16.
Hemorrhage was defined as a persistent intraparenchymal hyperdense area on the follow-up CT which did not resolve for more than 24 h after EVT.
All images were independently evaluated by two experienced investigators (J.Q and W. L) who were blinded to the clinical data. Disagreements between these investigators were reviewed by a third adjudicator (Z.A.Z), and were resolved by majority opinion.
Main outcomes
The primary outcomes of the study were to evaluate good and poor outcome, which was defined as mRS 0–2 and mRS 3–6 at 90 days after stroke, respectively.
Measurement reproducibility
To assess intrarater and interrater variability, two experienced investigators (J.Q and W. L) who were blind to the clinical data independently reassessed CE volume and CE -ASPECT of all patients 1 month later.
Statistical analysis
Continuous variables are expressed as the mean with SD, or median with interquartile range (IQR), and categorical variables were described as frequencies and percentages. Continuous variables were compared with the t-test or Mann–Whitney U test. The chi-square test or Fisher’s exact test was used to compare categorical variables. Multivariable analysis was performed using multiple logistic regression analysis to evaluate factors that may predict progression to poor outcome. We adjusted for the clinical outcome-related covariates reported in previous studies, including age, initial NIHSS, the procedure time, number of stent retriever passes and mTICI 2b-3. Multicollinearity of the independent variables were analyzed using the variance inflation factor (VIF) statistic. Interrater and intrarater variability of CE volume and CE-ASPECTS was tested with intraclass correlation coefficient (ICC) (two-way random, absolute agreement, single measures). All statistical analyses were conducted using IBM SPSS version 25.0 (IBM Inc), and a P value less than 0.05 was considered statistically significant.
Ethics approval
This retrospective study was approved by our institutional review board (IRB: Y (2020)087).
Results
We retrospectively included 158 consecutive anterior circulation AIS-LVO patients of which 113 (71.52%) had hyperdensity on NCCT within 1 h after EVT. We excluded patients with hemorrhage (n = 42), imaging not assessable (n = 2), incomplete clinical data (n = 3) and pre-stroke disability (mRS ≥ 2) (n = 2). Finally, the remaining 64 patients were enrolled for the current analysis (Fig. 2).
The baseline characteristics of the patients are demonstrated in Table 1. A total of 28 patients (44%) had good outcome and 36 patients (56%) had poor outcome. Patients with good clinical outcome had lower median initial NIHSS score (13 (± 4) vs. 15 (± 5); P = 0.046), higher CE-ASPECTS score (7 (±2) vs. 5 (±3); P = 0.031) and lower CE volume (5.89 (±8.51) vs. 33.35 (±65.16); P = 0.015). Higher prevalence of CE at M2, M4, M5, and M6 regions were noted in the poor outcome group (all P < 0.05). There were no significant differences in other factors between the two groups.
On univariable analysis, lower CE-ASPECTS score and higher CE volume were significantly associated with poor clinical outcome after EVT. Additionally, the presence of CE at the caudate nucleus, M2, M4, M5, and M6 region was also found to be related to poor outcome. Higher initial NIHSS score showed a tendency to poor prognosis (P = 0.051), but other factors failed to show a significant association (Table 2). In addition, the scores of CE-ASPECTS was associated with baseline ASPECTS of infarct lesions (ρ = 0.31; P < 0.05, Spearman correlation test).
On multivariable logistic regression analysis, CE-ASPECTS (aOR 0.80, 95% CI 0.65–0.98, P < 0.05), CE volume (aOR 1.27, 95% CI 1.01–1.61, P < 0.05, for every 5 ml increment in CE volume), CE at the caudate nucleus (aOR7.56, 95% CI 1.22–46.82, P < 0.05), CE at M4 region (aOR 6.09, 95% CI 1.55–23.99, P < 0.05), and M6 region (aOR 4.46, 95% CI 1.19–16.67, P < 0.05) were associated with 3-month poor functional outcome after adjusting for age, initial NIHSS, the procedure time, stent retriever passes and recanalization status (mTICI 2b-3) (Table 3). The VIF and tolerance were < 10 and > 0.1, respectively, suggesting no co-linearity among the final predictors (Supplementary Table I).
As shown in Fig. 3, we built several models to predict poor outcome. First, the conventional variables were used as model 1 for reference, including age, initial NIHSS, the procedure time, stent retriever passes, recanalization status and baseline ASPECTS, with the area under the receiver operator characteristic curve (AUC) of 0.73 (95% CI 0.61–0.86). When individually added to the predictive model based on conventional variables, CE volume (AUC 0.80, 95% CI 0.69–0.91), or CE-ASPECTS (AUC 0.78, 95% CI 0.67–0.89) improved discriminative performance. The location variables of CE, combination of CE at C, M4 and M6 regions, also had significant discriminative performance with an AUC of 0.85 (95%CI 0.76–0.94). When combined with the above-named variables (conventional variables + CE-ASPECTS + CE volume + CE at caudate nucleus + CE at M4 region + CE at M6 region), the predictive power was further improved, with the AUC of 0.87 (95% CI 0.78–0.95), significantly higher than model 1 (∆AUC of 0.13, p = 0.014).
Interrater reliability was excellent for both CE volume (ICC = 0.993; 95%CI 0.981–0.996; p < 0.001) and CE-ASPECTS (ICC = 0.995; 95%CI 0.991–0.997; p < 0.001). Intrarater ICC ranged from 0.993 (95%CI 0.987–0.997) to 0.994 (95%CI 0.988–0.996) for CE volume, and from 0.994 (95%CI 0.993–0.997) to 0.996 (95%CI 0.990–0.996) for CE-ASPECTS (Supplementary Table II).
Discussion
CE has been commonly found on the NCCT obtained immediately after EVT in patients with AIS3,4,5,6,7,8,9,10, which has been attributed to the disruption of the blood brain barrier4,11,12,13. In the present study, the incidence of CE on NCCT was 71.5%, which was comparable to prior reports (30.7–87.5%3,4,5,6,7,8,9,10). Our work is novel in that we adapted the methodology of ASPECTS score to CE combined with volume measurement to assess the extent and location of CE, and found that CE based on this quantitative method after EVT was an independent and strong predictor of clinical functional outcome after excluding definite hemorrhage by 24-h follow up CT.
Previous studies have investigated the relationship of the hyperdensity on NCCT and clinical outcomes, but the results were conflicting. Some studies found that the presence of a high-density lesion on NCCT had no prognostic value to clinical outcome8,9,10. In contrast, Portela et al.3 found that the total size of the hyperdense area had a positive correlation with 90-day mRS after EVT. As previously reported, the hyperdensity may be related to the contrast agent, hemorrhagic transformation or a combination9,11,13,17, which may be caused by varying degrees of BBB disruption. Based on the dual-energy CT (DECT) technology, which allows an accurate differentiation between contrast staining and hemorrhage, Renú et al.4 and Chen et al.5 reported that contrast staining within 24 h of EVT was associated with an increased risk of hemorrhage and was also an independent predictor of unfavorable clinical outcomes. The definitions of the hyperdensity in these studies were of two kinds: high attenuation and contrast staining, depending on whether it contains hemorrhage. We surmise that the controversial results of these studies may be related to the following reasons: the heterogeneous definitions of hyperdensity based on the different computed tomography techniques after EVT (high attenuation on NCCT3,8,9 vs. contrast staining on DECT4,5,10), or the lack of quantitative assessment (quantitative3 vs. non-quantitative4,5,8,9,10).
We adapted the methodology of ASPECTS score to CE combined with volume measurement by 3D reconstruction to ensure precise evaluation of the extent and location of CE on the NCCT obtained immediately after EVT. To our knowledge, this is the first report to describe this technique in the evaluation of CE after EVT. Although DECT is considered the gold standard of differentiating extravasation of contrast agent and cerebral hemorrhage after EVT, with high sensitivity and specificity4,5,18,19,20, it is not readily available in most stroke centres. In addition, given that hemorrhagic transformation was found to be associated with poor functional outcome in AIS-LVO patients in previous studies, especially PH2 (> 30% of the infarcted area with significant space-occupying effect)21,22,23, we explored the relationship of CE on NCCT with functional outcome after exclusion of cases with definite hemorrhagic transformation. Interestingly, the presence of CE in the caudate, M2, M4, M5, and M6 region were significantly related to poor outcome, which may be explained by the fact that these cortical regions of ASPECTS contained eloquent cerebral functional areas involving the pre-motor, motor and parietal cortex. Another possible explanation is that the presence of CE in cortical areas may be associated with poor collateral circulation, which was an independent predictor of poor clinical prognosis in acute ischemic stroke24,25,26,27,28. The other possibility may be associated with pre-existing early infarct prior to EVT, which was supported by the correlation between the scores of CE-ASPECTS and initial ASPECTS of infarct lesions in this study (ρ = 0.31; P < 0.05, Spearman correlation test). Of note, the head of the caudate nucleus manifested similar outcomes to the cortex structures, which could be explained by its shared supply from the anterior cerebral artery, the latter territory of which is often eloquent and not included in ASPECTS grading13,16,29.
Limitations
We acknowledge limitations in our study. A main limitation is that a 24-h follow-up CT after EVT is needed to exclude the hemorrhage in the current study, which will limit the clinical value of current findings. However, the current findings will be helpful for the patients whose contrast enhancement after EVT can be evaluated without the need for 24-h CT after EVT. On the other hand, the current findings will provide the important information for patients with contrast enhancement after EVT whose hemorrhage was excluded by early DECT. Second, the data based on a relatively small sample size and single medical center, were susceptible to selection bias. A larger, patient sample may further refine the model. Because of the retrospective nature of the present study, the potential confounders could not be completely controlled. Third, the dose of the contrast agent was not collected in this study due to the retrospective design. It was reported that high amounts of contrast agent could be a potential contributor to BBB disruption due to its toxicity5,11,30, and may lead to the neurological complications of contrast-induced encephalopathy30,31. In the current study, comparable procedure time and stent retriever passes between groups may indirectly exclude the effect of CE amount. Fourth, we did not record the number of microcatheter injection runs obtained, which is also known to be associated with contrast extravasation and intracranial hemorrhage32. Finally, given that the present study focused on the effect of CE on clinical outcome, the patients with definite hemorrhage on the immediate NCCT after EVT were excluded to avoid the confounding effect, thus our conclusion does not apply to patients with hemorrhagic transformation.
Conclusion
CE-ASPECTS and higher CE volume on NCCT obtained immediately after EVT in patients with AIS may be independently associated with poor functional outcome at 3-months after excluding definite hemorrhage by 24-h follow up CT. The predictive performance for poor outcome was higher when CE was located in the head of caudate nucleus, and the cortical M4 and M6 regions of ASPECTS.
Data availability
Data are available upon reasonable request.
References
Eskey, C. J. et al. Indications for the performance of intracranial endovascular neurointerventional procedures: a scientific statement from the American Heart Association. Circulation https://doi.org/10.1161/CIR.0000000000000567 (2018).
Lin, Y. et al. Endovascular thrombectomy as a means to improve survival in acute ischemic stroke: a meta-analysis. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2019.0525 (2019).
Portela de Oliveira, E. et al. Value of high-density sign on CT images after mechanical thrombectomy for large vessel occlusion in predicting hemorrhage and unfavorable outcome. Neuroradiol. J. https://doi.org/10.1177/1971400920975259 (2021).
Renu, A. et al. Relevance of blood-brain barrier disruption after endovascular treatment of ischemic stroke: Dual-energy computed tomographic study. Stroke https://doi.org/10.1161/STROKEAHA.114.008147 (2015).
Chen, Z. et al. Contrast extravasation is predictive of poor clinical outcomes in patients undergoing endovascular therapy for acute ischemic stroke in the anterior circulation. J. Stroke Cerebrovasc. Dis. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.104494 (2019).
Kim, H. et al. Subarachnoid contrast accumulation and Alberta Stroke Program Early Computed Tomography Score applied to contrast accumulation after thrombectomy as predictors of symptomatic hemorrhage. World Neurosurg. https://doi.org/10.1016/j.wneu.2020.03.102 (2020).
Song, S. Y. et al. Extent of contrast enhancement on non-enhanced computed tomography after intra-arterial thrombectomy for acute infarction on anterior circulation: As a predictive value for malignant brain edema. J. Korean Neurosurg. Soc. https://doi.org/10.3340/jkns.2015.58.4.321 (2015).
Lummel, N. et al. Hyperattenuated intracerebral lesions after mechanical recanalization in acute stroke. Am. J. Neuroradiol. https://doi.org/10.3174/ajnr.A3656 (2014).
Parrilla, G. et al. Hemorrhage/contrast staining areas after mechanical intra-arterial thrombectomy in acute ischemic stroke: Imaging findings and clinical significance. Am. J. Neuroradiol. https://doi.org/10.3174/ajnr.A3044 (2012).
An, H. et al. Contrast staining may be associated with intracerebral hemorrhage but not functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Aging Dis. https://doi.org/10.14336/AD.2018.0807 (2019).
Yoon, W. et al. Contrast enhancement and contrast extravasation on computed tomography after intra-arterial thrombolysis in patients with acute ischemic stroke. Stroke https://doi.org/10.1161/01.STR.0000120726.69501.74 (2004).
Cabral, F. B. et al. Hyper-attenuating brain lesions on CT after ischemic stroke and thrombectomy are associated with final brain infarction. Interv. Neuroradiol. https://doi.org/10.1177/1591019917729550 (2017).
Nakano, S. et al. Parenchymal hyperdensity on computed tomography after intra-arterial reperfusion therapy for acute middle cerebral artery occlusion: Incidence and clinical significance. Stroke https://doi.org/10.1161/hs0901.095602 (2001).
Barber, P. A. et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet https://doi.org/10.1016/s0140-6736(00)02237-6 (2000).
Demchuk, A. M. et al. Alberta Stroke Program Early CT Score in acute stroke triage. Neuroimaging Clin. N. Am. https://doi.org/10.1016/j.nic.2005.06.008 (2005).
Chen, M. et al. Comparison of common methods for precision volume measurement of hematoma. Comput. Math. Methods Med. https://doi.org/10.1155/2020/6930836 (2020).
Whitney, E. et al. Contrast extravasation post thrombectomy in patients with acute cerebral stroke: A review and recommendations for future studies. Cureus https://doi.org/10.7759/cureus.10616 (2020).
Almqvist, H. et al. Dual energy CT after stroke thrombectomy alters assessment of hemorrhagic complications. Neurology https://doi.org/10.1212/WNL.0000000000008093 (2019).
Liu, K. et al. The role of dual energy CT in evaluating hemorrhagic complications at different stages after thrombectomy. Front. Neurol. https://doi.org/10.3389/fneur.2020.583411 (2020).
Gupta, R. et al. Evaluation of dual-energy CT for differentiating intracerebral hemorrhage from iodinated contrast material staining. Radiology https://doi.org/10.1148/radiol.10091806 (2010).
Dzialowski, I. et al. Asymptomatic hemorrhage after thrombolysis may not be benign: Prognosis by hemorrhage type in the canadian alteplase for stroke effectiveness study registry. Stroke https://doi.org/10.1161/01.STR.0000251644.76546.62 (2007).
Van Kranendonk, K. R. et al. Hemorrhagic transformation is associated with poor functional outcome in patients with acute ischemic stroke due to a large vessel occlusion. J. NeuroInterv. Surg. https://doi.org/10.1136/neurintsurg-2018-014141 (2019).
Dekeyzer, S. et al. Distinction between contrast staining and hemorrhage after endovascular stroke treatment: One CT is not enough. J. NeuroInterv. Surg. https://doi.org/10.1136/neurintsurg-2016-012290 (2017).
Wabnitz, A. M. et al. Hemodynamic markers in the anterior circulation as predictors of recurrent stroke in patients with intracranial stenosis. Stroke https://doi.org/10.1161/STROKEAHA.118.020840 (2019).
Alves, H. C. et al. Associations between collateral status and thrombus characteristics and their impact in anterior circulation stroke. Stroke https://doi.org/10.1161/STROKEAHA.117.019509 (2018).
Al-Dasuqi, K. et al. Effects of collateral status on infarct distribution following endovascular therapy in large vessel occlusion stroke. Stroke https://doi.org/10.1161/STROKEAHA.120.029892 (2020).
Broocks, G. et al. Computed tomography angiography collateral profile is directly linked to early edema progression rate in acute ischemic stroke. Stroke https://doi.org/10.1161/STROKEAHA.119.027062 (2019).
Seners, P. et al. Better collaterals are independently associated with post-thrombolysis recanalization before thrombectomy. Stroke https://doi.org/10.1161/STROKEAHA.118.022815 (2019).
Kumral, E. et al. Acute caudate vascular lesions. Stroke https://doi.org/10.1161/01.str.30.1.100 (1999).
Chu, Y. T. et al. Contrast-nduced encephalopathy after endovascular thrombectomy for acute ischemic stroke. Stroke https://doi.org/10.1161/STROKEAHA.120.031518 (2020).
Vigano, M. et al. Contrast-induced encephalopathy mimicking total anterior circulation stroke: A case report and review of the literature. Neurol. Sci. https://doi.org/10.1007/s10072-020-04844-1 (2021).
Khatri, P. et al. Microcatheter contrast injections during intra-arterial thrombolysis may increase intracranial hemorrhage risk. Stroke https://doi.org/10.1161/STROKEAHA.108.522904 (2008).
Funding
The work was supported by the Science and Technology Plan of Shen Yang (20-205-4-007) and the Science and Technology Project Plan of Liao Ning Province (2018225023, 2019JH2/10300027).
Author information
Authors and Affiliations
Contributions
G.C.C. and D.C.M. retrospectively acquired the data. L.L., X.H.S., Y.G.Z., X.L., Z.A.Z. acquired the data. G.C.C. did statistical analysis and wrote the draft. W.L. and J.Q. analyzed imaging data. T.N.N. critically revised the manuscript. H.S.C. designed the study and critically revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chang, GC., Ma, DC., Li, W. et al. Contrast enhancement by location and volume is associated with long-term outcome after thrombectomy in acute ischemic stroke. Sci Rep 12, 16998 (2022). https://doi.org/10.1038/s41598-022-21276-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21276-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.