
Efflorescence is one of the first signs of moisture problems for cementitious materials, especially masonry. A by-product of moisture combining with free salts, this phenomenon is not only just a cosmetic problem—left unchecked during freeze-thaw conditions, it can cause brick to weaken, spall, or crumble in some cases.
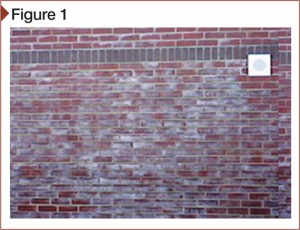
Depending on its source, efflorescence is usually a white powdery substance (Figure 1). It forms on different cementitious and clay construction when internal, ‘restless’ moisture dissolves free water-soluble salt deposits in the masonry and carries them to the surface (Figure 2). The moisture and salts can also promote galvanic action. When the moisture containing the dissolved salts evaporates, efflorescence appears.
The type of salt compound in the structure dictates the appearance—alkali salt compounds usually found in mortar and cement products (e.g. sodium or potassium) produce the more common whitish to light gray deposits, but there is a wide range of variation. Depending on the type of salt compound in the structure, it can also range from shades of brown and green; it can be powdery, crystalline, dull, hazy, or bright.
According to the Brick Institute of America (BIA) and at least one masonry cleaning/sealing product manufacturer, metallic salt compounds from vanadium, manganese chromium, and molybdenum tend to produce discoloration (i.e. stains) other than white or gray. These compounds are often used as a colorant in specialty brick.
Some discolorations are often referred to as ‘stains’ because they usually occur during chemical cleaning, are more difficult to remove, and can be permanent. For instance, vanadium is greenish, while manganese tends to be brown streaks resembling tobacco stains (Figure 3).
Lime run is another discoloration very similar to, and often confused with, efflorescence. It usually forms in the joints and runs down the face of the masonry units during cleaning (Figure 4). The salts and resulting colors are different, but the production processes are basically the same.
Efflorescence 101
Efflorescence can cover large surface areas, individual masonry units, or partial brick. These reactions bring the salts to the surface in a solution that forms crystals when it combines with carbon dioxide (CO2) in the air.

(B) Efflorescence caused by improper drainage and no waterproofing behind the retaining wall.
(C) An improper metal canopy tie-in directed moisture to the concrete lintel, causing efflorescence, spalling, and deterioration of attempted repairs.
(D) Efflorescence on shaded clay pavers over an unprotected base.
(E) Efflorescence mixed with traces of mildew on shaded concrete pavers caused by moisture migrating from the unprotected base.
(F) Moisture mixed with the salts in a clay pot planter that was kept in the shade and formed a heavy crust of efflorescence.
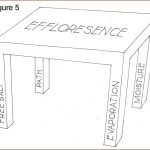
The term ‘efflorescence’ is not always properly pronounced (“eff-floor-ess-sense”), much less adequately understood by design/construction professionals. It is the result of the combination of four factors:
- salt deposits;
- moisture;
- moisture path to the surface; and
- evaporation.
As shown in Figure 5, it is analogous to a four-legged table where eliminating any one of the legs causes collapse. Remove one ‘leg’ and efflorescence is stopped.
Hidden efflorescence can be present in masonry when the salts are dissolved by moisture, but the moisture has not migrated to the surface. A rudimentary form of efflorescence can be made by sprinkling some table salt (i.e. sodium chloride) in a clear glass and adding water—the salt then dissolves. When the water is poured out, and the residual moisture in the glass evaporates, a cloudy white salt film is left on the glass (Figure 6).
The powdery form of efflorescence can usually be brushed away. However, when it is allowed to go through repeated cycles of being deposited, rain-dissolved, and re-dried, it becomes crystalline, tightly bonding to the surface.
Efflorescence is unsightly and is usually a source of disagreement between architects, designers, builders, and owners as to why it occurs and what should be done when it appears. Indeed, according to ASTM C1364, Standard Specification for Architectural Cast Stone, efflorescence alone on manufactured units is not a reason for rejection. Still, if allowed to progress, the result can be spalling that affects masonry’s structural and durability properties.
It is not always possible to predict whether efflorescence will occur. Soluble salt components may be present in concrete, mortar, brick, concrete masonry unit (CMU), cast stone, portland cement stucco, or earth. They may be carried into the wall with any form of precipitation, fog, condensation, sprinkler systems, or be absorbed by groundwater.














Thanks for informative item
I cleaned my patio and used poly sand and acrylic sealer, pavers turned white in shaded area of patio. Tried to get rid of white using xylene and did not work, some turned whiter
Hi did u ever find out wat it was and did u get it off
What can I do to remove the white powdery from the temptationsis rick
I have the same problem on a brick walkway???
I tried everything on my backyard brick paving. Then I remembered that engine oil dripping from the car permanently darkens brick paving. Painted my paving with engine oil and got the original colour of the bricks back. It absorbed immediately. Not affected by rain so far. Not sure what the long term will bring but looks good.
A good informative article.
I have the same problem please and i urgently need to overcome it. Please if there is a solution then kindly waiting for it.
I had an area of Redbrick wall which had been discoloured – I therefore Pressure washed it. The discolouration has gone but the pressure washed area is now white. How to remove the white ?
Ta
Use vinegar in a pump spray it works to remove the white deposit on the bricks
Do you dilute the vinegar?
I tried to use vinegar to spray on the red bricks
But it can not working,the white deposit still there.
You don’t have to dilute it but I have found if you stain the bricks with brick stain it will cover it up
Ugh, I used muriatic acid on a 93 degree day and I think I burned my red bricks , they are sooo white. What do I do now, stain the brick?
My bricks on a project are doing the same thing. Keep turning white. Washed with 100% Muronic acid . Brushed on, left for 5 minutes. When brick is wet, it turns dark red. When dry, white still returns.
Wheat else can be done. How do I stain brick red ?
Same problem there is something other than efflorescence going on here than simple. I tried almost everything and did notice that acid does not fizz on the white stuck to the red brick which tells me it is something else but it does fizz on the mortar joints which is bad if I continue to try and clean as I am dissolving the lime
Couple of things worth noting if your water used for the job and the cleaning has high calcium ie hard water we are just making it worse by wetting brick and the acid clean as you are soaking bad water in the brick this causing more efflorescence to occur after it dries and evaporates after cleaning the last lot lol. Also if you rinse and repeat you actually make the salt hardened instead so it’s just going to get worse. So by using water to soak the brick is actually not a good idea
Great article.Ive got efflorescence on the brickwork round my stove. Ive tried to remove it with white vinegar but its just as bad.Could it be the PVA sealer has burned into the brick? I’ll keep trying! Any suggestions? Cheers
i used muriatic acid, brushed it with broom. left it on over night
i had to repeat in some area. but now its all gone!!
not sure how long it will last.
Dr Nelson: great to hear your acid treatment worked. Did you pre-rinse the wall and rinse off again afterwards..? I ask because my acid washes and scrubbing isn’t doing much to remove and I’m tempted to splash the acid on neat and just leave it on the brickwork, in the hope it’ll eat into the white efflo.
Also, has anyone got a solution to the darkness appearance of the damp bricks of a retaining wall?! I’ve got patches of dark bricks because they (assumed) are always damp. I’m guessing this is unfixable.
I’m wondering if NEW brickwork should be SEALED with something to PREVENT efflorescence, but I don’t know what products are best.