Abstract
Groupers (Family Epinephelidae) are important to commercial, subsistence, and recreational fisheries throughout the world. Grouper species have complex life histories that make them more vulnerable to exploitation. While there has been extensive research on some, the majority of groupers lack sufficient life history information for proper management. The yellow-edge lyretail grouper, Variola louti, has life history gaps that need to be filled and lacks regional life history information for Guam. Age, growth, and reproduction were assessed from fishery-dependent samples collected from around Guam from 2010 to 2017. Variola louti ranged from 19.4 to 49.7 cm fork length (FL) and 2 to 17 years old. Due to the size selectivity of the fishery, a Bayesian von Bertalanffy growth model was applied. The von Bertalanffy growth parameters were L∞ = 43.7 cm FL, k = 0.28, and t0 = − 0.2. Protogynous hermaphroditism was confirmed with females reaching reproductive maturity at 26.0 cm FL (L50) and 2.6 years (A50) and female to male sex change at 35.3 cm FL (LΔ50) and 6.1 years (AΔ50). Sex ratio was 1.5 females per male, excluding transitional individuals. Using Hoenig’s method, natural mortality was estimated at 0.37 year−1. The life history of Variola louti suggests that it is a relatively fast growing and early maturing grouper that is not as vulnerable to exploitation as larger bodied, slower growing groupers. However, future monitoring of sex ratio, size at maturity, and size at sex change is recommended to track and manage fishery effects, such as the recent scuba spear ban in Guam, on the life history and population status of this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Groupers (Family Epinephelidae), further defined as the subfamily Epinephelinae, are currently considered to contain 9 genera (Alphestes, Cephalopholis, Dermatolepis, Epinephelus, Gonioplectrus, Hyporthodus, Mycteroperca, Plectropomus, and Variola) and are found throughout the world in many different ocean habitats (Ma and Craig 2018). Traditionally, grouper species were considered to be comprised of 15 genera; however, molecular phylogeny research suggests the 9 genera above which include 167 species (reviewed in Félix-Hackradt et al. 2022). Groupers exhibit a diverse array of life histories with some of the larger species reaching sexual maturity as late as 10 years of age, and longevity exceeding 50 years (Andrews et al. 2019; Craig et al. 2011). Groupers also exhibit a diverse array of reproductive patterns; however, they tend to exhibit monandric protogynous hermaphroditism (i.e., all males derive from adult females by sex change). Some exceptions to this hermaphroditic pattern exist which can include bisexual juvenile reproductive development, diandry (primary males and secondary males derived by sex change), and gonochorism (Sadovy and Colin 1995).
Groupers are a commercially important assemblage of species in several markets including the aquarium and live fish trades, as well as many recreational, commercial, and subsistence fisheries throughout the world. Unfortunately, their complex life histories can make them more vulnerable to exploitation (for review see Sadovy de Mitcheson et al. 2020). In general, grouper life history and reproductive biology traits result in low resilience to exploitation and increased vulnerability to overfishing (Coleman et al. 2000; Sadovy de Mitcheson et al. 2020), especially for those that are large-bodied, long-lived, slow-growing, and form spawning aggregations (Sadovy de Mitcheson et al. 2020). Despite this, the life histories and reproductive strategies of many species of groupers remain unknown, making efforts to effectively manage such species difficult.
The genus Variola is part of a monophyletic clade shared with genera Plectropomus and Gonioplectrus (Ma and Craig 2018). There are only two identified species within the genus Variola: Variola louti and Variola albimarginata. Variola spp. have a wide Indo-Pacific distribution and are found in coral reef, reef crest, and outer slope habitats at depths of 15–250 m (Nair et al. 2017). Reported maximum size is 65 cm fork length (FL) for V. albimarginata and 81 cm FL for V. louti (Craig et al. 2011), and prior research suggests that both are relatively short-lived (Grandcourt 2005; Nair et al. 2017). For example, Grandcourt (2005) reported a maximum size of 60 cm FL, a maximum age of 15 years, L∞ = 51.0 cm FL, and k = 0.48 for V. louti from Seychelles. Variola spp. are also believed to be hermaphroditic, but this has yet to be confirmed. Their reproductive strategy, including size and age at maturity and size and age at sex change, is currently unknown.
Variola louti are captured in multiple fisheries and with multiple gear types throughout their range (Galal et al. 2002; Grandcourt 2005). In Guam, V. louti are part of a shallow water reef fishery (predominantly spear fishing) and a shallow water bottomfish fishery (using hook and line in depths that are outside of spear and scuba depth limits and < 135 m). The minimum size reported is around 20.0 cm FL, and the maximum size previously reported is 49.0 cm FL (Kamikawa et al. 2015). Estimated annual landings are highly variable for this species around Guam ranging from 19 to 3159 kg (mean = 688 kg; Bohaboy and Matthews 2023). Management action in March 2020 saw the banning of spearfishing on scuba for all fish species in response to research which indicated the overall size, as well as the ratio of large to small individuals, had declined in Guam over the past 25 years (Houk et al. 2018). This highlights the need for more information on V. louti life history in order to better assess population status and future management strategies, in particular, the impact of the scuba-spear ban.
This study investigated the age, growth, natural mortality, and reproduction of Variola louti using biological samples collected from the reef and bottomfish fisheries in Guam from 2010 to 2017. This information fills life history gaps for the species and adds to the collective understanding of the biology of groupers in the Pacific which is an important component of population monitoring and sustainable fisheries management.
Methods
Sample collection
A biological sample collection program was established in Guam in 2010 (Sundberg et al. 2015). This Commercial Fisheries Biosampling Program collected fishery-dependent length observations (n = 1147) and biological samples (otoliths [n = 287] and gonads [n = 255]) of V. louti from the waters around Guam from 2010 to 2017. Additionally, during research surveys in summer 2014, five V. louti were caught in Asuncion, Maug, Pagan, and Uracus (Fig. S1), Commonwealth of the Northern Marianas Islands (CNMI). These fish were aged and compared to the ages of those from Guam. Ultimately, they were not included in the growth model because they came from locations that are considered lightly fished and may not be representative of fish in Guam. Comparisons between Guam and these lightly fished areas serve as a check on potential fishery effects on growth and length-at-age.
Length distributions of the biological samples (n = 287) were compared to the entire fishery-dependent length observations for V. louti (n = 1147) using a Kolmogorov–Smirnov test to determine if biological samples constituted a representative sample of the fishery. Additionally, a Student’s t test was performed to compare mean length from the biological samples to fishery-dependent length observations.
All fish were measured for FL (0.1 cm), weighed (W [g]), and gonads and otoliths extracted. Otoliths were cleaned, weighed (OW [0.001 g]), and stored in plastic vials. Gonads were weighed (GW [0.001 g]) and a midsection of the gonad from one of the lobes was removed and stored in 10% buffered formalin.
Fishery-dependent length observations were analyzed to determine if gear or fishing method (hook and line [shallow water bottomfish fishery] or freediving spear and scuba spear [reef fishery]) impacted length frequencies. A Kolmogorov–Smirnov test was used to determine if there were differences in V. louti length distributions between the bottomfish fishery and reef fishery. The percent of the samples from the shallow water bottomfish fishery and reef fishery were assessed to determine the fishery’s contribution of the field-dependent length data and biological samples.
Age and growth
One otolith (either right or left, randomly chosen) was examined for condition (i.e., not broken or chipped) and, if suitable, the sagitta was marked to identify the primordium on the medial surface along the sulcus acusticus. Each individual otolith was mounted and transversely sectioned perpendicular to the sulcus acusticus using a Buehler precision Isomet saw with two blades separated by a 400 µm spacer (Usseglio et al. 2015) or affixed by thermoplastic adhesive to a slide and ground along the primordium using a GEMMASTA GFL8 lapping wheel to a thickness of 400 µm (O’Malley et al. 2019). The otolith sections were then polished using lapping film (30 to 0.3 µm grit) to a thickness of 200–240 µm until the banding pattern was clearly visible. Otoliths were cover slipped with casting clear epoxy resin (Fiberglass Hawaii) before reading. Samples were read blind (i.e., no knowledge of FL, weight, or date of capture) and were read twice by a single reader with a minimum of two weeks between reads. Ages were accepted when two reads matched and read a third time if they were different. Two age readers read a subset reference collection of the Guam V. louti otoliths (n = 62) to determine average percent error (APE) and coefficient of variation (CV). The APE and CV were averaged across all individuals to estimate the Index of Average Percent Error (IAPE) and average CV. Age-bias plots were used to assess symmetry and identify any systematic differences in aging between readers (Campana et al. 1995). Edge type analysis (Newman and Dunk 2003) was done to validate increment periodicity and compared to mean monthly sea surface temperature from Guam (NOAA Coral Reef Watch 2022). Linear regression was used to determine the relationship between otolith weight and age.
A Bayesian von Bertalanffy growth model was used with the following model (von Bertalanffy 1938):
where Lt is the predicted mean length-at-age t (years), L∞ is the asymptotic FL in cm, k is the growth coefficient, t is the estimated age in years, L0 is the length at hatch at theoretical time zero (t0), and σ is the model dispersion/error. Theoretical time zero \({(t}_{0}\)) is calculated from L∞, k, and L0 using the equation:
where a normally distributed prior was used for L∞ and set to the maximum size encountered in biological samples (50 cm), a half normal prior (truncated at 0) was used for L0, and a uniform prior was used for k and σ following methods by Smart and Grammer (2021). Markov Chain Monte Carlo (MCMC) was used to sample the posterior distributions for each growth parameter. Four chains were used with a 1000 iteration warm up (burn in) and 4000 total post-warmup draws with a thin equal to 1. Posterior distribution means and 95% credible intervals (CI) were reported.
Bayesian model fit was assessed with prior sensitivity analyses and posterior predictive distributions, where observed values were compared to the posterior distributions (McElreath 2018; Vehtari et al. 2017). Well-calibrated models should include the observation within the high density of the distribution (McElreath 2018). Trace plots, R-hat, and effective sample size were checked for model convergence (McElreath 2018). Pareto smoothed importance sampling leave-one-out (PSIP-LOO) was used to determine if there were any high-leverage observations (Pareto \(\widehat{k}\) estimate > 0.7). See Supplemental Information for prior and posterior model checks.
Natural mortality (M) was estimated using Hoenig et al. (1983) updated method (Then et al. 2015), as follows:
where tmax = maximum age (17 years).
Reproduction
Gonad samples were embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin counterstaining at John A. Burns Medical School Histopathology Core Facility at the University of Hawaii. Female and male reproductive phases were assigned following criteria by Brown-Peterson et al. (2011) (Table 1). Regenerating females were differentiated from immature females by having two or more diagnostic criteria of prior spawning activity (e.g., thick ovary walls, atretic oocytes, post ovulatory follicles, muscle bundles, brown bodies, or enlarged blood vesicles). Gonads that contained both ovarian and testicular tissue were classified as transitional or as a functional male with primary oocytes (male with PO) (Sadovy De Mitcheson and Liu 2008; Schemmel et al. 2016). Lastly, hermaphroditic pattern was diagnosed based on Sadovy and Shapiro (1987).
The two criteria used to assess female reproductive maturity were (1) physiological maturity (L50p): presence of cortical alveoli, and (2) functional maturity (L50f): onset of vitellogenesis (Brown‐Peterson et al. 2011). Because there is no universal standard for maturity criteria (Lowerre-Barbieri et al. 2011), both physiological and functional maturity were assessed to allow for comparisons to prior and future studies.
Size (L50) and age (A50) at sexual maturity (i.e., the size or age at which 50% of females are mature), and size (LΔ50) and age (AΔ50) at female to male sex change were assessed using a logistic regression model with binomial family and logit link function with 2 cm length bins for size and 1 year bins for age (Chen and Paloheimo 1994). Estimates of L50 and A50 were generated using 1000 bootstrapped replicates of the model coefficients. Likelihood ratio tests were used to determine if size or age at maturity differed based on physiological or functional maturity criteria.
Gonadosomatic index (GSI) was assessed from histologically identified females to determine reproductive investment with size. GSI was assessed using the following equation:
Mean monthly GSI was assessed from functionally mature females (n = 106) and males (n = 86) with gonad weights. Mean monthly GSI and the proportion of females in spawning capable and actively spawning reproductive phases per month were used to identify the spawning season. The relationship between GSI and lunar day was also assessed using a generalized additive model (GAM). Spawning fraction was estimated by assessing the frequency of individuals spawning using the hydrated oocyte method (DeMartini and Fountain 1981). Lastly, spawning interval (i.e., the time period between spawning events) was estimated as the inverse of the spawning fraction (Lowerre-Barbieri et al. 2011).
All analyses and statistical tests were performed in R (R Core Team 2020) using the packages mgcv, FSA, and Stan (Stan Development Team 2023).
Results
Demographics
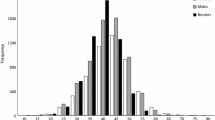
The majority (76%) of fishery-dependent lengths (n = 1147) was measured from the reef fishery (95% scuba spear; 5% spear), while the shallow water bottomfish fishery (hook and line) accounted for 21% of lengths, and the remaining 3% were categorized as a mixture of both shallow water bottomfish and reef fishing methods. Fishery-dependent length distributions of V. louti differed between the reef and shallow water bottomfish fishery (D = 0.28, p value < 0.01; Fig. 1). V. louti were, on average, 4.2 cm FL larger from the reef fishery compared to the shallow water bottomfish fishery (t = − 11.23, df = 390.81, p value < 0.01). Similarly, median size of V. louti from the reef fishery was 3.4 cm larger than the shallow water bottomfish fishery.
A total of 289 otoliths and 255 gonads were sampled from the waters around Guam. The majority of V. louti biological samples were from the reef fishery (59%); the shallow water bottomfish fishery accounted for 39%, and 2% did not have a gear type or fishery specified. Across all individuals, 146 females, 54 males, 42 males with primary stage oocytes, and 13 transitional individuals (both ovarian and spermatogenic tissue present but inactive) were histologically identified. The male (males and males with PO) to female sex ratio was 40.0%. Fork lengths ranged from 19.4 to 49.7 cm for the biological samples and 19.8 to 53.6 cm for the fishery-length observations. The length distributions of biological samples and fishery-length observations did not differ (D = 0.06, p value = 0.18) and mean lengths did not differ (t = 1.12, df = 326.87, p value = 0.27), indicating that biological samples collected were representative of the fishery.
Age, growth, and mortality
Opaque marks were visible on otoliths polished to a thickness between 200 and 240 µm (Fig. 2b). Opaque marks were presumed to be deposited annually, the highest proportion of which were deposited December through March which coincided with the lowest mean monthly sea surface temperatures (Fig. 2a). There was a strong relationship between age and otolith weight (Fig. 2b; y = 0.009 + 0.009*Age, R2 = 0.88, p value < 0.01). Between reader agreement was 45%, and agreement within 1 year was 84%. The average CV was 8.5% and the IAPE was 6.0%. The age-bias plot shows slight disagreement between readers for individuals over 11 years (Fig. 3).
Edge analysis showing proportion of Variola louti otoliths with opaque margin (solid line) and mean sea surface temperature by month (dashed line) for fish sampled from Guam between 2010 and 2017, and b relationship between otolith weight and age (n = 247) of V. louti from Guam (black circles) and CNMI (white triangles). Plotted line is a linear regression fit to the data (y = 0.009 + 0.009*Age, R.2 = 0.88, p value < 0.01). Inset image is a V. louti polished otolith showing opaque marks (age = 12 years) from a 39.1 cm FL female with 1 mm scale bar (inset image is enlarged in Supplemental Material Fig. S2)
Ages of V. louti ranged from 2 to 17 years. Males were larger (7.4 cm, t = − 13.16, p value < 0.01) and older (2.5 years, t = − 8.21, p value ≤ 0.01) than females (Fig. 4). The maximum age was 12 years for females and 17 years for males. The mean age was 4.7 years for females and 7.1 years for males. There was significant variability in length-at-age (Fig. 5; see Supplemental Information for table of means and CV). The estimated von Bertalanffy growth function parameters (95% CI) were k = 0.28 (0.24–0.32), L∞ = 43.7 cm FL (42.0–45.8 cm FL), and t0 = − 0.2 years (− 0.5–0.0) (Table 2; Fig. 5). The length-at-age of the fish from CNMI fell within the range of fish from Guam (Fig. 5). As expected for size selected sex change for protogynous hermaphrodites, sex-specific growth was observed with males reaching a larger L∞ than females (Supplemental Information Table S3 and Fig. S5); however, we focus on combined sex growth parameters as these are comparable to other studies. Natural mortality estimated using Hoenig’s method was 0.37 years−1.
Percent frequency of Variola louti reproductive stages by fork length (cm) and age (years) for fish sampled from Guam between 2010 and 2017. Developing stages (Developing I & Developing II) were grouped together into “Developing.” Male reproductive phases are grouped into “Male.” Males with PO (primary oocytes) are separated into their own group regardless of the reproductive phase (developing or spawning capable)
Bayesian von Bertalanffy growth curve for Guam Variola louti (L∞ = 43.7 [42.0–45.8], k = 0.28 [0.24–0.32], and t0 = − 0.2 [− 0.5–0.0]) sampled between 2010 and 2017. Black line is mean and dashed lines are 95% credible intervals. Females (grey circles), transitional (squares), males (males and males with primary oocytes; black circles), and CNMI individuals of unknown sex (triangles) which were not included in the growth model estimation
Reproduction
There was clear evidence that V. louti are protogynous hermaphrodites. Length and age distributions differed between sexes (Fig. 4) and histology revealed the presence of transitional individuals (Fig. 6). The presence of a central lumen in the testes and the fact that the smallest males are larger and older than the smallest transitional individuals further support monandric protogynous hermaphroditism. The smallest transitional individual was 25.6 cm FL and the youngest was 3 years (Fig. 4). The smallest male with primary oocytes was 27.5 cm (age unknown) and as young as 4 years. The smallest male (without primary oocytes) was 29.1 cm and as young as 5 years.
Transitional and male reproductive phases of Variola louti: a transitional phase with degenerating vitellogenic oocytes (A), a few remnant hydrated oocytes (H), and spermatogonia (SG) and spermatocytes (SC); b male with PO primary stage oocytes (PO), lumen (L), melanomacrophage centers (MMC), and spermatogonia and spermatocytes; c, d spawning capable male with PO with developed sperm ducts with spermatozoa (SZ), scattered primary stage oocytes throughout the gonad; and e, f spawning capable male with sperm sinuses full of spermatozoa and melanomacrophage centers scattered throughout the gonad. Scale bars in all panels are 250 μm
Gonad histology showed a transition from a female functioning ovary to a male functioning testis (Figs. 6 and 7). The transition from female to male appears to begin in the regressing reproductive phase with the appearance of spermatogenic tissue in the form of small pockets of spermatogonia and spermatocytes scattered throughout the gonad and intermingled with ovarian tissue (Fig. 6a, b). One transitional individual had a few remaining hydrated oocytes, several degenerating vitellogenic oocytes, and small pockets of spermatocytes (Fig. 6a). Primary oocytes appeared to remain in the testes of developing and spawning capable males for extended periods as the lumen shrinks and spermatogenesis progresses (Fig. 6d).
Female reproductive phases of Variola louti: a, b undeveloped female ovary with primary oocytes (PO) and tunica (T); c spawning capable female with PO, cortical alveolar (CA), vitellogenic II (VTII), and vitellogenic (VTIII) oocytes; d actively spawning female with hydrated oocytes (H); e regressing female with atretic vitellogenic oocytes (A), space in the lumen (L), and large mussel bundles (MB); and f regenerating female with PO and large blood vessels (BV). Scale bars in all panels are 250 μm
Female GSI was influenced by reproductive phase as well as fish size (Fig. 8a). Mean (± SE) GSI of females was 0.11 (± 0.02) for undeveloped, 0.37 (± 0.70) for developing, 1.99 (± 0.28) for spawning capable, 0.35 (± 0.09) for regressing, 0.16 (± 0.01) for regenerating, and 3.12 (± 0.70) for actively spawning phases. Spawning capable and actively spawning females had variable GSI across fish sizes (Fig. 8a). There was a weak relationship between reproductive output (i.e., log gonad weight) and gonad free body weight (Fig. 8b; log GW = 1.3860 + GFBW * 0.0016, df = 40, R2 = 0.23).
a Female Guam Variola louti gonadosomatic index (GSI) versus fork length, and b spawning capable and actively spawning female log gonad weight (GW) versus gonad free body weight (GFBW) (log GW = 1.3860 + GFBW * 0.0016, df = 40, R2 = 0.23) for fish sampled from Guam between 2010 and 2017. Spawning capable and actively spawning females are black, immature females are white, and all other female reproductive phases are gray
Spawning capable females were encountered throughout the year (Fig. 9). GSI peaked from November through March (Fig. 10a). However, spawning appeared to occur most of the year. Spawning peaked during the first quarter and last quarter of the lunar phase (F value = 3.865, edf = 5.331, rdf = 6.428, p value < 0.01; Fig. 10b). The spawning fraction (i.e., the number of actively spawning females out of the total number of mature females) was 0.11 which translated to a spawning interval of approximately 9 days.
Female size at maturity (L50) was estimated physiologically at 25.8 cm FL (CI: 25.4–27.1 cm FL) and functionally at 26.0 cm FL (CI: 25.6–27.3 cm FL) (Table 2, Fig. 11). Physiological age at maturity was 2.4 years (CI: 2.1–3.1 years) and functional age at maturity was 2.6 years (CI: 2.4–3.1 years) (Table 2, Fig. 11). Female size and age at physiological maturity were not significantly different from functional maturity (L50: χ2 = 0.042, df = 1, p value = 0.84 and A50:χ2 = 0.031, df = 1, p value = 0.86). Size at sex change was 35.5 cm FL (CI: 35.1–36.4 cm FL) and age at sex change was 6.1 years (6.1–6.7 years) (Table 2; Fig. 11).
Variola louti a size at functional maturity (L50 = 26.0 cm FL), b age at functional maturity (A50 = 2.6 years), c size at sex change (LΔ50 = 35.5 cm FL), and (d) age at sex change (AΔ50 = 6.1 years) for fish sampled from Guam between 2010 and 2017. Solid lines are logistic regression fit to the data and dashed lines are 95% confidence intervals. Sample size is indicated by the size of the grey circles
Discussion
While Variola louti are present in many fisheries around the tropics, little was known about the species’ life history and vulnerability to exploitation. In this study, Variola louti exhibited moderate growth (k = 0.28) and reach L∞ at 43.7 cm FL within 8–10 years. Protogynous hermaphroditism was confirmed for this species; females reach reproductive maturity at a small size (26.0 cm FL) and young age (2.6 year), and female to male sex change occurs, on average, at 35.3 cm FL and 6.1 years of age. These life history characteristics suggest that V. louti are not as vulnerable to exploitation compared to larger, slower growing hermaphroditic groupers such as Galapagos grouper (Mycteroperca olfax, Usseglio et al. 2015), Hawaiian grouper (Hyporthodus quernus, Andrews et al. 2019; DeMartini et al. 2011), and Nassau grouper (Epinephelus striatus, Sadovy and Eklund 1999).
There is debate on the best approach to deal with juveniles missing from von Bertalanffy growth models (Kritzer et al. 2001; Pardo et al. 2013) because small changes in the value used to fix t0 can cause large changes in the other von Bertalanffy growth parameters k and L∞ (Pardo et al. 2013; Schemmel et al. 2022). A Bayesian growth model with informed priors was used to improve model fit under minimum size selectivity in the fishery (absence of juveniles under 20.0 cm in the sample). This approach allowed the setting of the L0 prior to a biologically reasonable range. An alternative approach to deal with minimum size selectivity is to fix or constrain t0 = 0 or fix t0 to larval size at hatching (Prince et al. 2015). Grandcourt (2005) fixed t0 = 0 for V. louti from the Seychelles, with a resulting k = 0.48, L∞ = 51.0 cm FL, and max length of 60.0 cm FL. For Guam samples herein, when t0 was fixed at 0, k was increased to 0.31 and L∞ was reduced to 42.8 cm FL. Using t0 fixed to the larval size at hatching, Prince et al. (2015) incorporated 5 studies on V. louti and V. albimarginata, including Grandcourt (2005), to model life history parameters for V. louti in Palau. Parameter estimates reported by Prince et al. (2015) were L∞ = 48.3 cm and L50/L∞ = 0.59. Our study’s estimate of L∞ is within the 95% CI reported by Prince et al. (2015), and the estimate of L50/L∞ was the same (0.59). Notably, recent research revealed that when juveniles are missing from the growth curve, constraining t0 = 0 is problematic unless a clear asymptotic length has been reached (Schemmel et al. 2022). Furthermore, there are no estimates of larval size at hatching in Guam. In the case of V. louti from Guam, the Bayesian approach helped overcome the low numbers of juveniles encountered and the lack of larval size at hatching information.
When large individuals are missing from the von Bertalanffy growth model, a clear asymptote in growth is not obtained. Our uncertainty in L∞ is demonstrated by the larger credible intervals around L∞. While additional older individuals may have helped the fit of the growth model, our sample selection for age and growth was proportional to the fished population. This is an important distinction, because overrepresentation of the largest, oldest individuals can also bias growth parameters (Schemmel et al. 2022).
The estimates of maximum age obtained in this study (17 years) are comparable to similar-sized hermaphroditic grouper species. Maximum ages of groupers range from 8 years for the small-bodied leopard coral grouper (Plectropomus leopardus; Payet et al. 2020) to 76 years (age validated to 50 years) for the large-bodied Hawaiian grouper (Hyporthodus quernus; Andrews et al. 2019). Compared to V. louti, the peacock grouper (Cephalopholis argus) has a similar geographic range, size (maximum size 49.6 cm FL), and age (maximum age of 16.7 years) (Donovan et al. 2013). Similarly, the squaretail common grouper (Plectropomus areolatus; maximum size 80 cm TL) has a reported maximum age of 10 years in Micronesia (Rhodes et al. 2021) and 14 years in the eastern Torres Strait (Williams et al. 2008).
The occurrence of transitional individuals observed through histological examination of the gonad tissue confirms protogynous hermaphroditism in V. louti. The frequency of females and males in the population suggests that the type of hermaphroditic pattern is monandric protogynous hermaphroditism. However, diandry, or the existence of both primary and secondary males, is not uncommon in protogynous grouper species, including Cephalopholis, Epinephelus, and Plectropomus species (Adams 2003; Chan and Sadovy 2002; Ebisawa 2013; Fennessy and Sadovy 2002; Liu and de Mitcheson 2009; Liu and Sadovy 2004; Rhodes et al. 2021; Sadovy and Colin 1995). Unfortunately, there were no individuals under 19.4 cm FL with which to determine early gonadal development. However, given the very low frequency of small, young males, diandry is unlikely.
Spawning appeared to occur all year in Guam V. louti, as spawning capable or actively spawning females were present in every month. Spawning peaked from November through March; however, the assessment of spawning seasonality is also likely influenced by sample size, sampling location, and the time of sampling. A semi-lunar spawning pattern was observed with peaks occurring during first and last quarter moons. Notably, lunar (full moon) and semi-lunar (new moon) spawning is common in groupers. A few examples include Plectropomus leopardus which spawn on new moons (Ebisawa 2013), P. areolatus which spawn on full and new moons (Rhodes et al. 2021), and Epinephelus fuscoguttatus which spawn on full moons (Pet et al. 2005). Some groupers do not follow a lunar spawning pattern, such as Mycteroperca jordani which spawn most of the month and can for spawn 15 consecutive days (Rowell et al. 2019). While not as common as new and full moon spawning, quarter moon spawning is observed in other groupers and other reef fish species (Rahman et al. 2000; Park et al 2006; Murata et al. 2022). The spawning timing of V. louti herein may be specific to Guam, as there can be intraspecific differences in spawning timing (Schemmel and Friedlander 2017; Fukunaga et al. 2020). In some instances, different studies have reported opposite lunar spawning patterns within the same species, for example, P. areolatus spawn on either new moons or full moons (for review see Rhodes et al. 2021). The lunar spawning pattern of a species may depend on the environmental, current, and tidal conditions experienced by the population. Variola louti seem to prefer the outer reef or slope habitat which may be heavily influenced by environmental conditions (Emslie et al. 2017); however, they can also be found in deeper coastal waters (< 135 m).
Female reproductive output was highly variable and was not strongly correlated with fish size or weight. This may be due to the influence of seasonal and lunar spawning patterns that were not accounted for in GSI analyses. This may also be due to the high rates of sex change observed, where larger females had reduced reproductive output (egg production) and presumably reallocated energy into sexual transition.
Size/age at maturity and size/age at sex change are important indicators of population productivity. This study found V. louti to reach maturity at 26.0 cm FL (24.3 cm standard length [SL]). Only one other study examined V. louti size at maturity and estimated L50 as 29.0 cm SL (~ 33 cm FL); however, assessment of maturity was done using macroscopic (as opposed to histological) methods (Loubens 1980). The differences in size at maturity of V. louti from that study (New Caledonia) and ours could stem from differences in methodology, regional environmental conditions, habitat, or exploitation rates. Variola louti age at maturity was comparable to other similar-sized protogynous groupers. Variola louti matured at 2.5 years compared to C. argus which matured at 1.2 years (Schemmel et al. 2016) and P. areolatus which matured at 2.9 years (Rhodes et al. 2021). Variola louti size and age at sex change was 35.5 cm FL and 6.1 years, respectively. The ratio of size at sex change to L∞ was within the range of other similar-sized grouper species (V. louti = 0.81, C. argus = 0.79, P. leopardus = 0.98); however, the ratio of age at sex change to maximum age was slightly lower (V. louti = 0.38, C. argus = 0.66, P. leopardus = 0.55). It is unclear how long the transitional, inactive, and reproductive phases last for V. louti. The prevalence of primary stage oocytes in functioning males suggests full oocyte degeneration is a lengthy process. However, males with primary oocytes appeared to be spawning capable. Primary oocytes have been seen in spawning capable males of other protogynous grouper species including C. argus (Schemmel et al. 2016). Future research to monitor size and age at sex change and size at maturity would be beneficial for monitoring shifts in population productivity, as shifts in size at sex change can indicate fishery effects.
Additionally, sex ratio and reproductive behavior are important indicators of exploitation vulnerability in hermaphroditic groupers. Of particular concern for protogynous groupers is the fishery removal of large individuals since most of them are male. This can lead to skewed sex ratios, eventual sperm limitation, and population collapse (Alonzo and Mangel 2004; Coleman et al. 1996). Many grouper species are known to form large, predictable spawning aggregations which make them easily targeted by fishers (Sadovy De Mitcheson et al. 2008; Sadovy De Mitcheson et al. 2020). Hermaphroditic groupers that form spawning aggregations can experience large shifts in sex ratios due to exploitation, with the percent of males declining to as low as 1% of the population (Coleman et al. 1996; Usseglio et al. 2015). However, sex ratios in hermaphroditic groupers can also be influenced by fishing gear type (Wyanski et al. 2000), spatial variability in demographic parameters (Lowerre-Barbieri et al. 2020), and reproductive behavior (Lowerre-Barbieri et al. 2020). Variola louti are described as roving serranids, meaning that they are often observed roving or patrolling their habitat (Pears 2005). This type of behavior differs from what has been described for several Cephalopholis spp. that are more cryptic and sedentary, exhibiting defined territories with several residing females (Schemmel et al. 2016). The relatively high proportion of males in the Guam population (40%; this study) and 30% in New Caledonia (Loubens 1980) indicates that V. louti are unlikely to form group spawning aggregations. Observations of V. louti reproductive behavior are needed to better understand the reproductive biology of the species and its vulnerability to fishery exploitation (Biggs et al. 2021).
The life history information presented here is invaluable for fisheries management and monitoring the impact of the management actions such as scuba spearfishing bans. The Guam scuba spearfishing ban may offer significant protection for V. louti as the majority of the catch of large individuals comes from scuba spear fishing in relatively shallow (< 40 m) water. This management action may go a long way toward reversing the declines in average fish size that Houk et al. (2018) documented in Guam. The relatively small size (26.0 cm FL) and young age (2.6 years) at maturity, and the relatively high proportion of males (40%) suggest that V. louti are considerably less vulnerable to exploitation than slower-growing and late-maturing groupers. Furthermore, this research fills critical knowledge gaps in growth and reproduction for this species and supports a collective understanding on the biology of groupers.
Data availability
Data and metadata are available at Pacific Islands Fish Sci.Center. 2021. Life History Program Life History Estimates, https://inport.nmfs.noaa.gov/inport/item/59002. Data analysis and models are available at https://github.com/MARVLS/Fish-Gonad-Staging/tree/main/analyses/Variola_louti_Schemmel_2023.
References
Adams S (2003) Morphological ontogeny of the gonad of three plectropomid species through sex differentiation and transition. J Fish Biol 63(1):22–36
Alonzo SH, Mangel M (2004) The effects of size-selective fisheries on the stock dynamics of and sperm limitation in sex-changing fish. Fish Bull 102:1–13
Andrews AH, DeMartini EE, Brodziak J, Nichols RS, Humphreys RL Jr (2019) Growth and longevity of Hawaiian grouper (Hyporthodus quernus)—input for management and conservation of a large, slow-growing grouper. Can J Fish Aquat 76(10):1874–1884
Biggs CR, Heyman WD, Farmer NA, Kobara SI, Bolser DG, Robinson J, Lowerre-Barbieri SK, Erisman BE (2021) The importance of spawning behavior in understanding the vulnerability of exploited marine fishes in the US Gulf of Mexico. PeerJ 9:e11814
Bohaboy EC, Matthews T (2023) Evaluation of the data available for bottomfish stock assessments in Guam. NOAA Tech Memo NMFS-PIFSC
Brown-Peterson NJ, Wyanski DM, Saborido-Rey F, Macewicz BJ, Lowerre-Barbieri SK (2011) A standardized terminology for describing reproductive development in fishes. Mar Coast Fish 3(1):52–70
Campana SE, Annand MC, McMillan JI (1995) Graphical and statistical methods for determining the consistency of age determinations. Tran Am Fish Soc 124(1):131–138
Chan TT, Sadovy Y (2002) Reproductive biology, age and growth in the chocolate hind, Cephalopholis boenak (Bloch, 1790). Hong Kong Mar Freshw Res 53(4):791–803
Chen Y, Paloheimo J (1994) Estimating fish length and age at 50% maturity using a logistic type model. Aquat Sci 56(3):206–219
Coleman FC, Koenig CC, Collins LA (1996) Reproductive styles of shallow-water groupers (Pisces: Serranidae) in the eastern Gulf of Mexico and the consequences of fishing spawning aggregations. Environ Biol Fish 47(2):129–141
Coleman FC, Koenig CC, Huntsman GR, Musick JA, Eklund AM, McGovern JC, Sedberry GR, Chapman RW, Grimes CB (2000) Long-lived reef fishes: the grouper-snapper complex. Fisheries 25(3):14–21
Craig MT, Sadovy de Mitcheson Y, Heemstra PC (2011) Groupers of the world. A field and market guide. CRC Press, Boca Raton, Florida, p 424
DeMartini EE, Fountain RK (1981) Ovarian cycling frequency and batch fecundity in the queenfish, Seriphus politus: attributes representative of serial spawning fishes. Fish Bull 79(3):547–560
DeMartini EE, Everson AR, Nichols RS (2011) Estimates of body sizes at maturation and at sex change, and the spawning seasonality and sex ratio of the endemic Hawaiian grouper (Hyporthodus quernus, F. Epinephelidae). Fish Bull 109:123–134
Donovan MK, Friedlander AM, DeMartini EE, Donahue MJ, Williams ID (2013) Demographic patterns in the peacock grouper (Cephalopholis argus), an introduced Hawaiian reef fish. Environ Biol Fish 96(8):981–994
Ebisawa A (2013) Life history traits of leopard coralgrouper Plectropomus leopardus in the Okinawa Islands, southwestern Japan. Fish Sci 79(6):911–921
Emslie MJ, Cheal AJ, Logan M (2017) The distribution and abundance of reef-associated predatory fishes on the Great Barrier Reef. Coral Reefs 36:829–846
Félix-Hackradt FC, Hackradt CW, García-Charton JA (2022) Biology and ecology of groupers. CRC Press, Boca Raton, Florida, p 296
Fennessy ST, Sadovy Y (2002) Reproductive biology of a diandric protogynous hermaphrodite, the serranid Epinephelus andersoni. Mar Freshw Res 53(2):147–158
Fukunaga K, Yamashina F, Takeuchi Y, Yamauchi C, Takemura A (2020) Moonlight is a key entrainer of lunar clock in the brain of the tropical grouper with full moon preference. BMC Zoology 5:1–13
Galal N, Ormond RFG, Hassan O (2002) Effect of a network of no-take reserves in increasing catch per unit effort and stocks of exploited reef fish at Nabq, South Sinai. Egypt Mar Freshw Res 53(2):199–205
Grandcourt E (2005) Demographic characteristics of selected epinepheline groupers (family: Serranidae; subfamily: Epinephelinae) from Aldabra Atoll, Seychelles. Atoll Res Bull 539(531): 199–216 https://doi.org/10.5479/si.00775630.539.199
Hoenig JM, Lawing WD, Hoenig NA (1983) Using mean age, mean length and median length data to estimate the total mortality rate. ICES CM 500(23):1–11
Houk P, Cuetos-Bueno J, Tibbatts B, Gutierrez J (2018) Variable density dependence and the restructuring of coral-reef fisheries across 25 years of exploitation. Sci Rep 8(1):5725. https://doi.org/10.1038/s41598-018-23971-6
Kamikawa K, Cruz E, Essington T, Hospital J, Brodziak J, Branch T (2015) Length–weight relationships for 85 fish species from Guam. J Appl Ichthyol 31(6):1171–1174
Kritzer JP, Davies CR, Mapstone BD (2001) Characterizing fish populations: effects of sample size and population structure on the precision of demographic parameter estimates. Can J Fish Aquat 58(8):1557–1568. https://doi.org/10.1139/f01-098
Liu M, de Mitcheson YS (2009) Gonad development during sexual differentiation in hatchery-produced orange-spotted grouper (Epinephelus coioides) and humpback grouper (Cromileptes altivelis) (Pisces: Serranidae, Epinephelinae). Aquaculture 287(1–2):191–202
Liu M, Sadovy Y (2004) Early gonadal development and primary males in the protogynous epinepheline. Cephalopholis Boenak J Fish Biol 65(4):987–1002
Loubens G (1980) Biologie de quelques espèces de Poissons du lagon néo calédonien. II. Sexualité et reproduction. Cahiers de 1’Indo-pacifique 2(1):41–72
Lowerre-Barbieri SK, Brown-Peterson NJ, Murua H, Tomkiewicz J, Wyanski DM, Saborido-Rey F (2011) Emerging issues and methodological advances in fisheries reproductive biology. Mar Coast Fish 3(1):32–51. https://doi.org/10.1080/19425120.2011.555725
Lowerre-Barbieri S, Menendez H, Bickford J, Switzer TS, Barbieri L, Koenig C (2020) Testing assumptions about sex change and spatial management in the protogynous gag grouper, Mycteroperca microlepis. Mar Ecol Prog Ser 639:199–214
Ma KY, Craig MT (2018) An inconvenient monophyly: an update on the taxonomy of the groupers (Epinephelidae). Copeia 106(3):443–456
McElreath R (2018) Statistical rethinking: A Bayesian course with examples in R and Stan. CRC Press, Boca Raton, Florida, p 593
Murata R, Amagai T, Izumida D, Mushirobira Y, Nozu R, Soyano K (2022) Lunar-related maturation and spawning migration in the honeycomb grouper, Epinephelus merra. Galaxea J Coral Reef Stud 24:31–88
Nair RJ, Samoilys M, Cabanban AS (2017) Variola louti, Yellow-edge Lyretail. IUCNNewman SJ, Dunk IJ (2003) Age validation, growth, mortality, and additional population parameters of the goldband snapper (Pristipomoides multidens) off the Kimberley coast of northwestern Australia. Fish Bull 101:116–128
Newman SJ, Dunk IJ (2003) Age validation, growth, mortality, and additional population parameters of the goldband snapper (Pristipomoides multidens) off the Kimberley coast of northwestern Australia. Fish Bull 101:116–128
NOAA Coral Reef Watch (2022) NOAA coral reef watch version 2.12 monthly sea surface temperature (CRW_sst_v1_0_monthly) data from Guam from 2010–01–01 to 2017–12–31. https://oceanwatch.pifsc.noaa.gov/erddap/griddap/CRW_sst_v1_0_monthly.html. Accessed 13 June 2022
O’Malley JM, Wakefield CB, Oyafuso ZS, Nichols RS, Taylor B, Williams AJ, Sapatu M, Marsik M (2019) Effects of exploitation evident in age-based demography of 2 deepwater snappers, the goldeneye jobfish (Pristipomoides flavipinnis) in the Samoa Archipelago and the goldflag jobfish (P. auricilla) in the Mariana Archipelago. Fish Bull 117(4):322–336. https://doi.org/10.7755/fb.117.4.5
Pardo SA, Cooper AB, Dulvy NK (2013) Avoiding fishy growth curves. Methods Ecol Evol 4(4):353–360
Park Y-J, Takemura A, Lee Y-D (2006) Annual and lunar-synchronized ovarian activity in two rabbitfish species in the Chuuk lagoon. Micronesia Fish Sci 72:166–172
Payet SD et al (2020) Comparative demography of commercially important species of coral grouper, Plectropomus leopardus and P. laevis, from Australia’s great barrier reef and Coral Sea marine parks. J Fish Biol 97(4):1165–1176
Pears RJ (2005) Comparative demography and assemblage structure of serranid fishes: implications for conservation and fisheries management. PhD thesis, James Cook University, Townsville
Pet JS, Mous PJ, Muljadi AH, Sadovy YJ, Squire L (2005) Aggregations of Plectropomus areolatus and Epinephelus fuscoguttatus (groupers, Serranidae) in the Komodo National Park, Indonesia: monitoring and implications for management. Environ Biol Fish 74(2):209–218
Prince J, Victor S, Kloulchad V, Hordyk A (2015) Length based SPR assessment of eleven Indo-Pacific coral reef fish populations in Palau. Fish Res 171:42–58
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Rahman MS, Takemura A, Takano K (2000) Correlation between plasma steroid hormones and vitellogenin profiles and lunar periodicity in the female golden rabbitfish, Siganus guttatus (Bloch). Comp Biochem Physiol Part B Biochem Mol Biol 127:113–122
Rhodes KL, Baremore IE, Taylor BM, Cuetos-Bueno J, Hernandez D (2021) Aligning fisheries management with life history in two commercially important groupers in Chuuk Federated States of Micronesia. Aquat Conserv 31(3):605–619
Rowell TJ, Schärer MT, Appeldoorn RS (2019) Description of a new sound produced by Nassau grouper at spawning aggregation sites. Gulf and Caribbean Research 29(1):GCFI22–GCFI26
Sadovy Y, Eklund AM (1999) Synopsis of biological data on the Nassau grouper, Epinephelus striatus (Bloch, 1792), and the jewfish, E. itajara (Lichtenstein, 1822). US Department Commer NOAA Technical Report NMFS 146, FAO Fisheries Synopsis 157, Seattle, Washington, pp 65
Sadovy Y, Colin P (1995) Sexual development and sexuality in the Nassau grouper. J Fish Biol 46(6):961–976
Sadovy Y, Shapiro DY (1987) Criteria for the diagnosis of hermaphroditism in fishes. Copeia 1987:136–156
Sadovy de Mitcheson Y, Liu M (2008) Functional hermaphroditism in teleosts. Fish Fish 9(1):1–43
Sadovy de Mitcheson Y, Cornish A, Domeier M, Colin PL, Russell M, Lindeman KC (2008) A global baseline for spawning aggregations of reef fishes. Conserv Biol 22(5):1233–1244
Sadovy de Mitcheson YJ et al (2020) Valuable but vulnerable: Over-fishing and under-management continue to threaten groupers so what now? Mar Policy 116:103909
Schemmel EM, Friedlander AM (2017) Participatory fishery monitoring is successful for understanding the reproductive biology needed for local fisheries management. Environ Biol Fish 100:171–185
Schemmel E, Donovan M, Wiggins C, Anzivino M, Friedlander A (2016) Reproductive life history of the introduced peacock grouper Cephalopholis argus in Hawaii. J Fish Biol 89(2):1271–1284
Schemmel E, Bohaboy E, Kinney M, O’Malley JM (2022) An assessment of sampling approaches for estimating growth from fishery-dependent biological samples. ICES J Mar Sci 79:1497–1514
Smart JJ, Grammer GL (2021) Modernising fish and shark growth curves with Bayesian length-at-age models. PLoS ONE 16(2):e0246734
Stan Development Team (2023) R Stan: the R interface to Stan. R package version 2.21.8, https://mc-stan.org.
Sundberg M, Humphreys R, Lowe MK, Cruz E, Gourley J, Ochavillo D (2015) Status of life history sampling conducted through the commercial fisheries biosampling programs in the Western Pacific Territories of American Samoa and Guam and in the Commonwealth of the Northern Mariana Islands Pacific Islands Fish Sci Cent, Natl Mar Fish Serv. Pacific Islands Fish Sci Cent, NOAA, Honolulu, HI 96818–5007. Pacific Islands Fish Sci Cent Admin Rep H-15–08: 1–56. https://doi.org/10.7289/V5XD0ZP5
Then AY, Hoenig JM, Hall NG, Hewitt DA, Handling editor: Ernesto Jardim (2015) Evaluating the predictive performance of empirical estimators of natural mortality rate using information on over 200 fish species. ICES J Mar Sci 72(1):82–92
Usseglio P, Friedlander AM, DeMartini EE, Schuhbauer A, Schemmel E, de Léon PS (2015) Improved estimates of age, growth and reproduction for the regionally endemic Galapagos sailfin grouper Mycteroperca olfax (Jenyns, 1840). PeerJ 3:e1270
Vehtari A, Gelman A, Gabry J (2017) Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat Comput 27(5):1413–1432
von Bertalanffy L (1938) A quantitative theory of organic growth (inquiries on growth laws. II). Hum Biol 10(2):181–213
Williams AJ, Currey LM, Begg GA, Murchie CD, Ballagh AC (2008) Population biology of coral trout species in eastern Torres Strait: implications for fishery management. Cont Shelf Res 28(16):2129–2142
Wyanski DM, White DB, Barans CA (2000) Growth, population age structure, and aspects of the reproductive biology of snowy grouper, Epinephelus niveatus, off North Carolina and South Carolina. Fish Bull 98:199–218
Acknowledgements
The Pacific Islands Fish Sci. Center Life History Program and Stock Assessment Program, in particular, Joseph O’Malley, Michael Kinney, and Erin Bohaboy for their thoughtful review of the manuscript. Corey Wakefield for review of aging criteria and reading a subset of the otoliths. The Guam Commercial Fisheries Biosampling Program for sample collection and processing of a subset of the otoliths, in particular Eric Cruz. Mary Donovan for statistical training through the Bayesian Statistical Modeling course (GIS 591) at Arizona State University. This project was supported by grants from the National Institute on Minority Health and Health Disparities (U54MD007601) National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Eva Schemmel was the lead on the research design, analysis, and writing. Kristen Dahl assisted in the aging of otoliths and reviewed the completed manuscript.
Corresponding author
Ethics declarations
Ethics approval
Biological sampling followed Institutional Animal Care and Use Standards under University of Hawaii IACUC (Protocol number 13–1696-8).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schemmel, E., Dahl, K. Age, growth, and reproduction of the yellow-edged lyretail Variola louti (Forssakal, 1775). Environ Biol Fish 106, 1247–1263 (2023). https://doi.org/10.1007/s10641-023-01411-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-023-01411-3